Developing transformative
medicines for people living
with microvascular disease.
Microvascular focus
Rationale

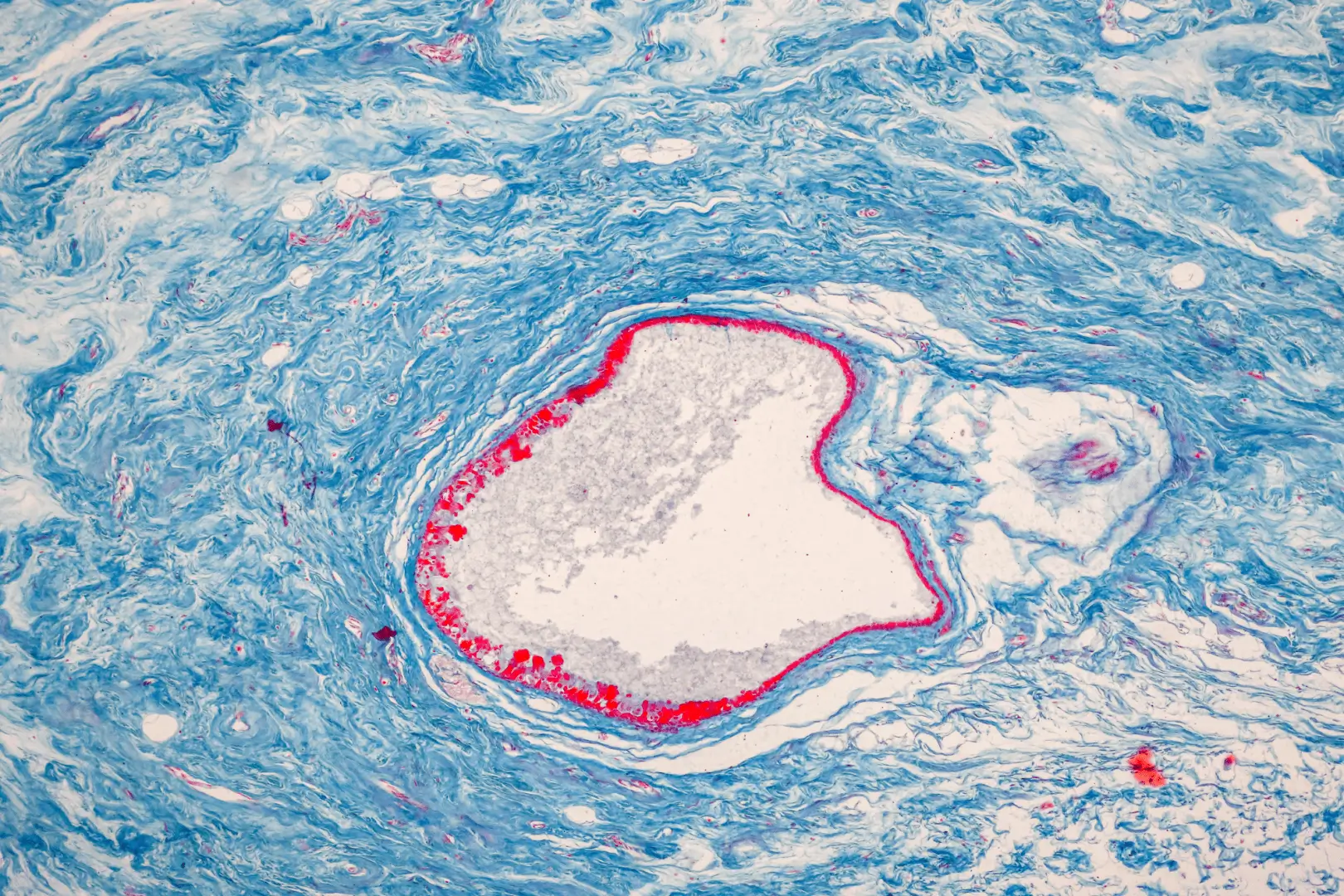
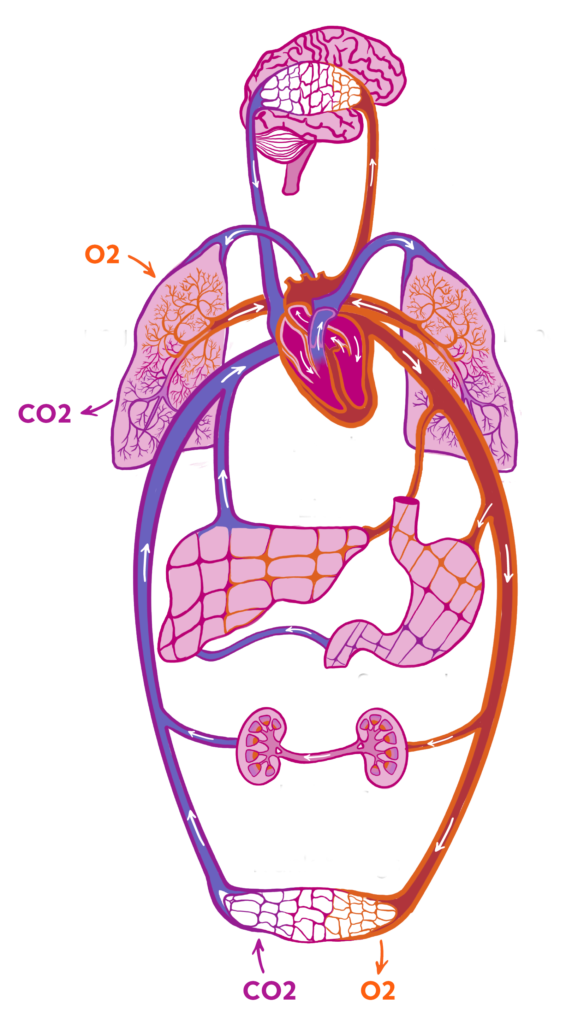
Tissue Perfusion
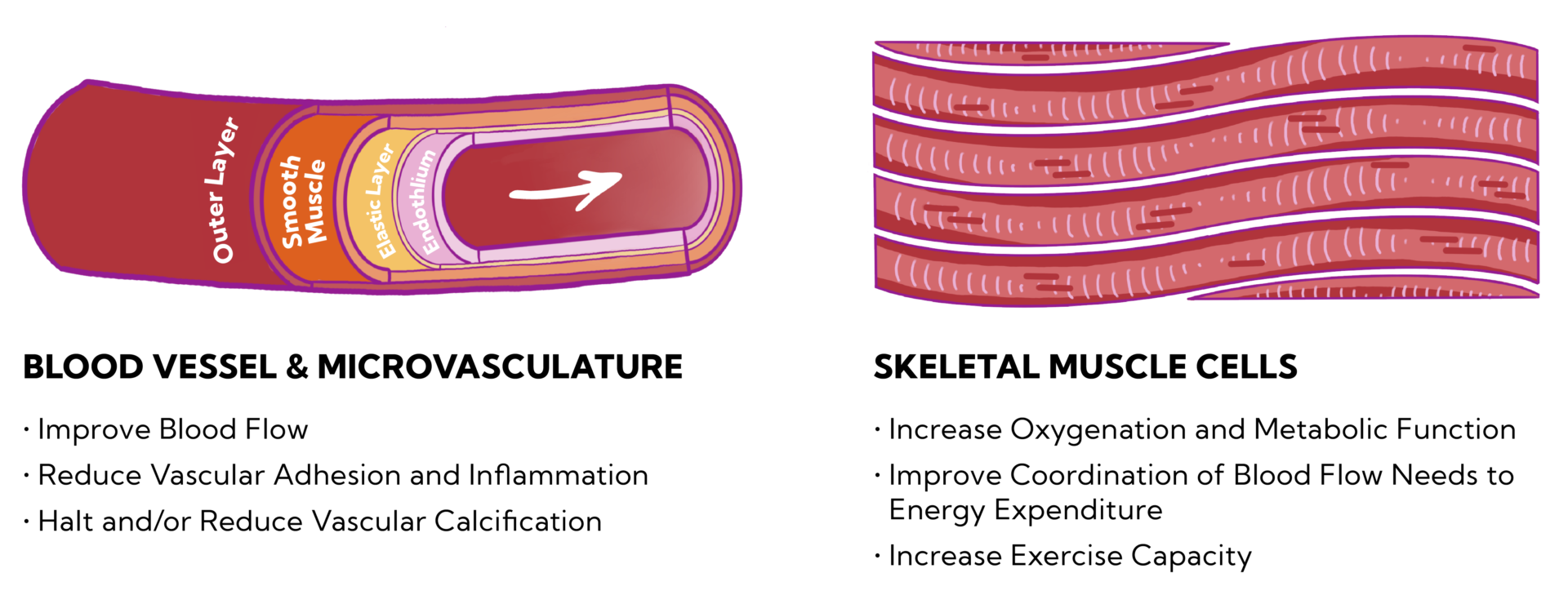
Core hemodynamic measures have traditionally focused on blood pressure and cardiovascular output. These measurements imperfectly reflect the tissue perfusion in organs.
Outsized Impact of Demographic Changes
In patients with cardiovascular risk factors such as diabetes, obesity, and even normal aging, structural and functional abnormalities in the microvessels are commonly observed
Technology
Microvascular assessment methods are still limited but rapidly advancing. Genetics and ML/AI enabled technology has potential to improve patient identification and management.

Optimization
Optimizing the microvascular structure and function may substantially improve the CV risk stratification.
Our pipeline is diversified across mechanisms of action
PROGRAM
MECHANISM
INDICATION
PRE-CLINICAL
PHASE 1
PHASE 2
PHASE 3
PROGRAM
Olinciguat* 1x Oral
MECHANISM
sGC stimulator
INDICATION
Microvascular Inflammatory Dysfunction
Coronary microvascular disease
PROGRAM
PROGRAM
MECHANISM
hd Menaquinone-7
INDICATION
Calciphylaxis